
 Product Name: Meningococcal vaccine
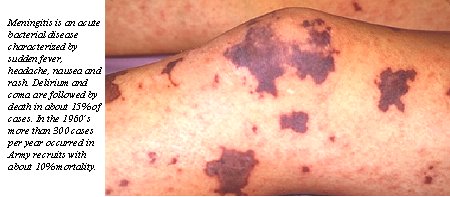
Product Name: Meningococcal vaccineCommercial Name: MENOMUNE Date of Licensure: A, C, Y, W-135, tetravalent vaccine licensed November 1981 Type of Product: purified capsular polysaccharide bacterial vaccine Company of Manufacture: Connaught Target microorganism/associated disease: Neisseria meningitidis is a gram-negative bacterium with the microscopic appearance of two kidney beans aligned along their long axis. The bacteria are encapsulated by a polysaccharide coat, which contains important determinant of potential virulence. Five antigenically distinct capsular polysaccharide serogroups, designated groups A, B, C, Y, and W-135, account for almost all human disease, although 13 groups have been identified. The bacteria are transmitted via infected respiratory droplets under conditions favoring close person-to-person contact. Humans serve as the only host and reservoir for N. meningitidis infections. After an incubation period of 2-10 days, the bacteria may either harmlessly colonize the human nasopharynx, or become invasive pathogens causing life-threatening (10-30% case-fatality rate) bacterial meningitis and sepsis. Whether a given meningococcal infection will result in an asymptomatic carrier state or in a devastating systemic disease depends on host immunity, bacterial virulence and other factors (lack of a spleen, cigarette smoking, viral upper respiratory infections and alcohol consumption are associated with increased risk of disease). Infections with N. meningitidis bacteria occur globally. Large-scale epidemics afflict Africa particularly in the sub-Saharan region during the dry season. The risk of meningococcal disease transmission to pilgrims on the hajj pilgrimage to Mecca in Saudi Arabia (and subsequent contacts of the pilgrims) has been recognized for decades. Meningococcal infections cause significant morbidity among pediatric populations, with the highest incidence in children under the age of 5 years, but there is a second peak in disease incidence among teenagers and young adults. Reason for development: Outbreaks of meningococcal disease among military recruits and soldiers have been documented since the civil war. Increased risks of meningococcal infections are associated historically with close exposures to large populations of persons from diverse geographic areas who are under stress. During the period from 1964 to 1971, coincident with the Vietnam War, an epidemic of group B and group C meningococcal disease occurred in U.S. military basic training centers. Approximately 2400 cases occurred in active duty military personnel annually with a case-fatality rate of about 7.2% (about 170 deaths). This epidemic, and the loss of sulfadiazine as an effective prophylactic agent (due to outbreak strain resistance) led to an intensive effort at the Walter Reed Army Institute of Research (WRAIR) to develop a vaccine to protect military personnel. Role of DoD in product development: Beginning in 1963, a team of investigators, led by Malcolm Artenstein, Emil Gotschlich, and Irving Goldschneider conducted studies over a 5 year period that elucidated the human immunologic response to meningococcus, including the concept that circulating antibody prevented clinical disease, and provided a basis for the successful development of effective polysaccharide vaccines for serogroups A and C. The C vaccine began to be used for routine vaccination of recruits entering basic training in 1970, and was replaced by an A/C divalent vaccine in 1978. The team's contributions included development of methods for purification of high molecular weight polysaccharides from groups A, B, and C; physical and chemical analysis of the polysaccharides; immunogenicity studies in several animal species; production of clinical grade vaccine; and phase 1, phase 2 and phase 3 clinical trials which demonstrated high efficacy and culminated in the licensure of the vaccine. Work on the development of vaccines for serogroups Y and W135 and testing of a tetravalent A, C, Y, W135 vaccine took place independently at several institutions including Connaught Laboratories, Inc., WRAIR, Institut Merieux, and the Rixensart, Belgium branch of Smith-Kline between 1975 and 1981. The group Y and W135 capsular polysaccharides were purified and characterized by these different institutions using the same methods developed at the WRAIR for the group C and A vaccines. The licensure of the tetravalent vaccine by Connaught Laboratories Inc. in 1981 was based on the company's own clinical data. Group B meningococcal vaccine is currently under development at WRAIR. |
| Influenza | Rubella Vaccine | Adenovirus | Meningococcal | Hepatitus B | Oral Typhoid | Japanese encephalitis | Hepatitis A |
