
Product Name: Rubella Vaccine Commercial Name: Meruvax (now Meruvax II) Date of Licensure: 1969 Type of Product: live-attenuated viral vaccine Company of Manufacture: Merck Target Microorganism/Associated Disease: Rubella, or German measles, is caused by a single-stranded RNA virus. The virus spreads person-to-person (humans are the only known host) via respiratory droplets. The virus causes fever and rash, which is followed by long-term joint pain and inflammation in rare cases. However, in pregnant women rubella infection can result in serious fetal malformations (hearing impairments, heart defects/inflammation, mental retardation, brain inflammation, enlargement of the spleen and liver, low blood platelets, etc.) particularly if acquired during the first two months of gestation.  Reasons for Development: Prior to 1969, military recruits and military hospital workers (particularly females of childbearing age) were at risk for rubella infection. Beginning in 1947, pregnant women exposed to rubella began receiving convalescent serum or immune serum globulin to prevent fetal infection. In 1962, a rubella pandemic struck Europe, which impacted the U.S. by 1962-63. This pandemic produced 12.5 million rubella cases and 2,000 cases of encephalitis in the U.S., and affected 30,000 infants (1% of all pregnancies, with 6,250 spontaneous abortions and 2,100 excess neonatal deaths). 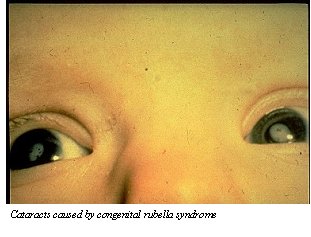 Role of Department of Defense in Vaccine Development: In 1962, COL Edward Buescher, COL Malcolm Artenstein, and CPT Paul Parkman of the Walter Reed Army Institute of Research (WRAIR) isolated the rubella (German measles) virus from a recruit who was hospitalized at Fort Dix, using virological techniques pioneered by scientists at WRAIR. (Weller and Neva also isolated the rubella virus at Harvard University in 1962). Parkman then moved on to the National Institutes of Health, where, together with Dr. Harry Meyer, he used the isolated virus to make an enduring form of the rubella vaccine. (A different viral strain [RA 27/3] was used as the basis of Meruvax II beginning in 1979.) The impact of the rubella vaccine has been dramatic: in the 3 years before the vaccine was brought to market in 1969, 47,745 cases of rubella were recorded in the U.S. alone; in comparison, only 345 cases were reported in 1998. |
| Influenza | Rubella Vaccine | Adenovirus | Meningococcal | Hepatitus B | Oral Typhoid | Japanese encephalitis | Hepatitis A |
